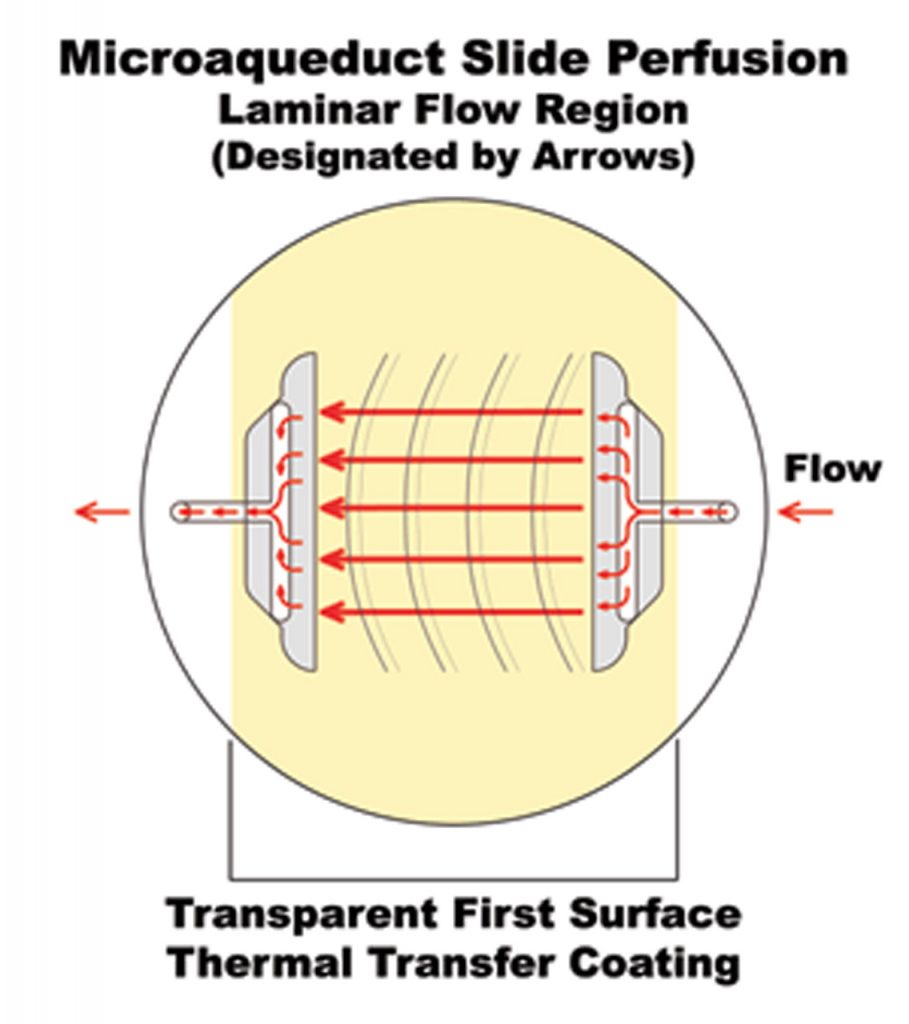
As Identified in drawing below #5 the Microaqueduct slide enables the laminar flow perfusion of fluid within the FCS2 and FCS3 Chamber systems.
Exploded View of FCS2 Chamber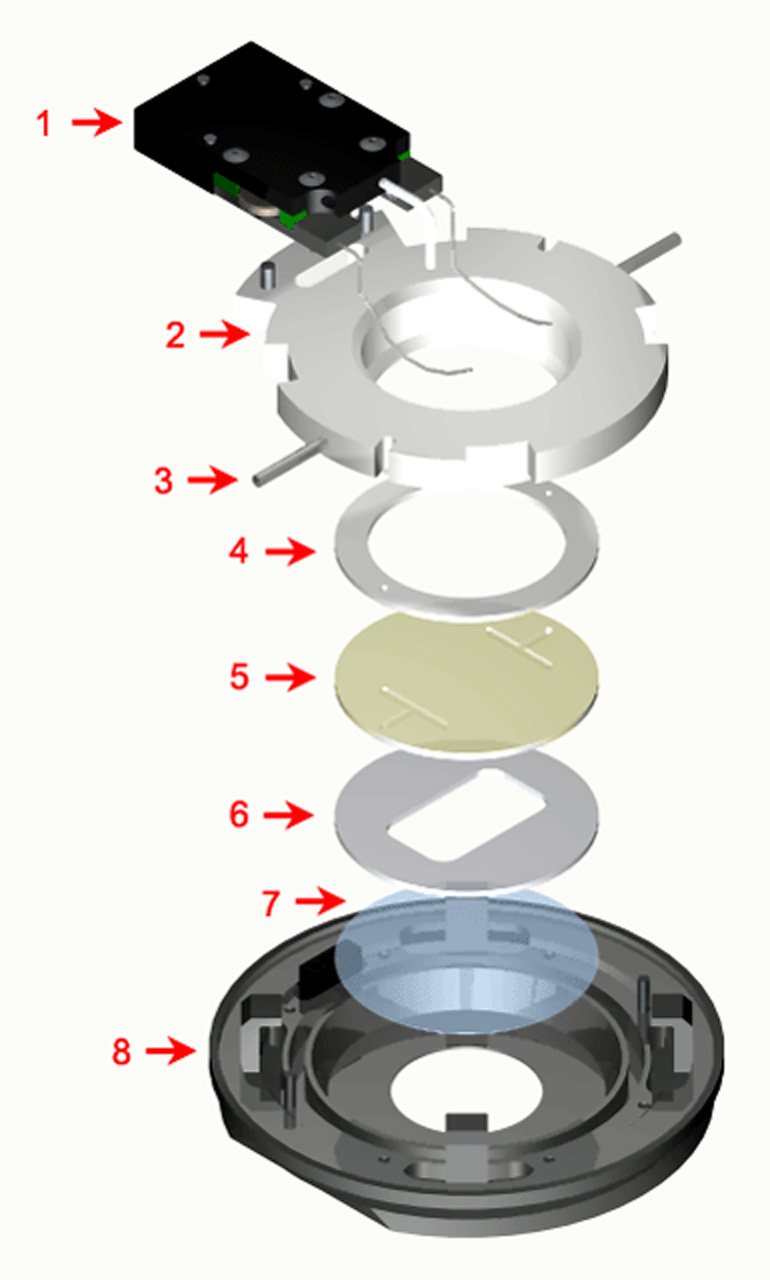
Items 5,6 & 7 are the optical cavity, the gasket (#6) can be changed to any one of the gaskets below or a custom gasket can be made to make any flow geometry, or media volume you want. The coverslip comes in a standard 0.17mm, #1.5 thickness but is also available in 0.5mm, #5 coverslip.
5 Pack of micro-aqueduct slides
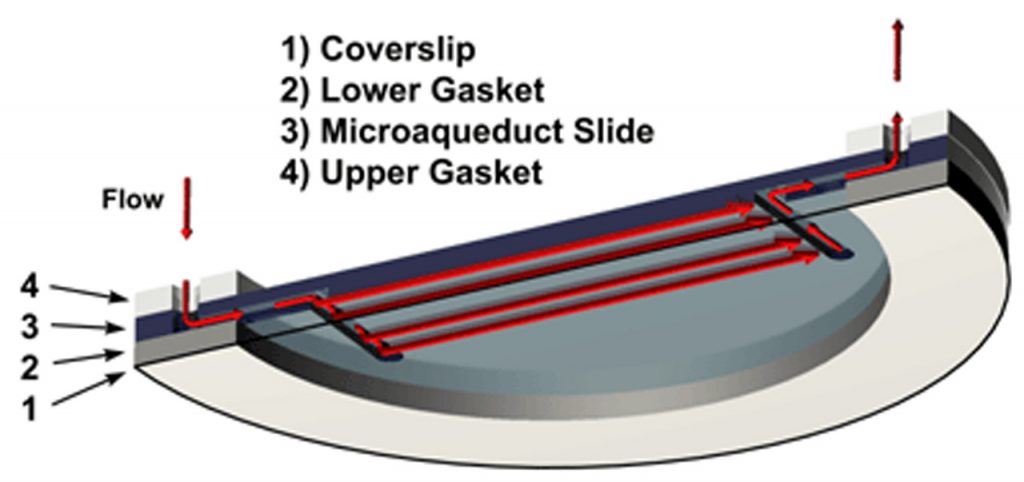
A fluid pathway is formed by separating the Microaqueduct Slide from the coverslip containing cells with a single silicone gasket. This gasket can be any thickness from 50 micron to 1mm and any lateral geometry you choose or create. This arrangement allows the user to define the flow characteristics. Therefore, you are not limited by the geometry of the optical cavity. Instead you select or create it! Fluid access to this flow channel is made through two 14-gauge needle stock tubes protruding from the sides of the chamber top. These tubes provide fluid connection to two perfusion holes in the Microaqueduct Slide that interface two “T” shaped grooves cut into the inner surface of the Microaqueduct Slide. The “T” groove allows the media to seek the path of least resistance and become nearly laminar before flowing across the cells. This technique eliminates the need for the metal perfusion ring and additional gaskets, which are the limiting factors, required by most conventional chambers.

3560 Beck Road
Butler, PA 16002
U.S.A.
sales@bioptechs.com
1-877-877-LIVE-CELL (548-3235)
(United States & Canada)
Direct: +1-724-282-7145
(United States & International)