AE: Mammalian Neuronal Monolayers Undergoing Electro Field Stimulation
AE: Mammalian Neuronal Monolayers Undergoing Electro Field Stimulation

I need to do fluorescence microscopy of mammalian, neuronal monolayers that are undergoing electro field stimulation at physiological temperatures in a nourishing environment. Is there a way to create the conditions to conduct this experiment while having direct access to the specimen?
PI at Harvard University
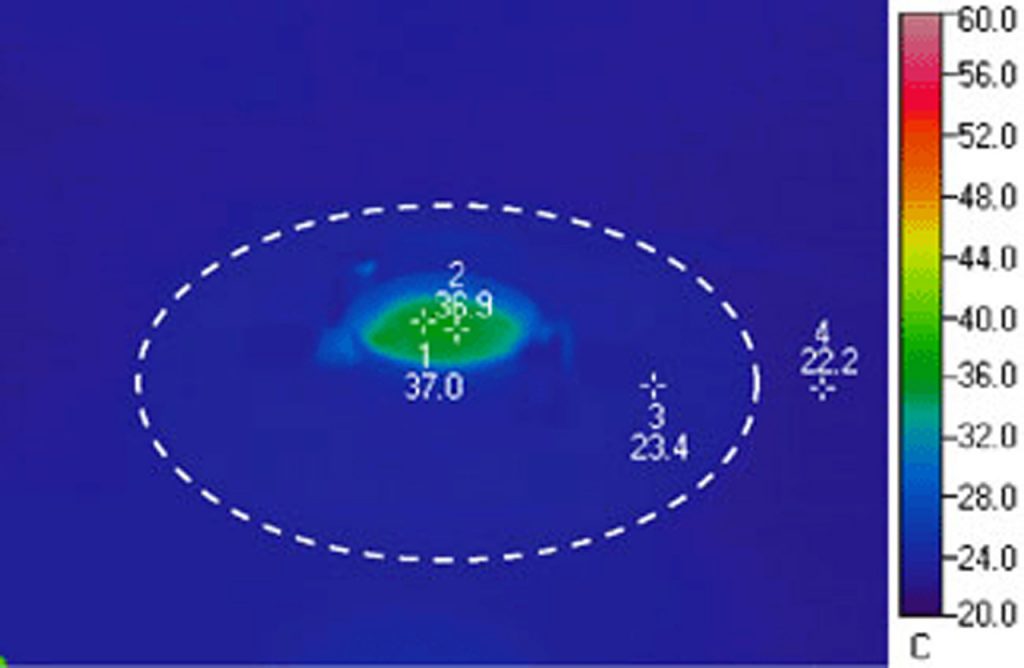
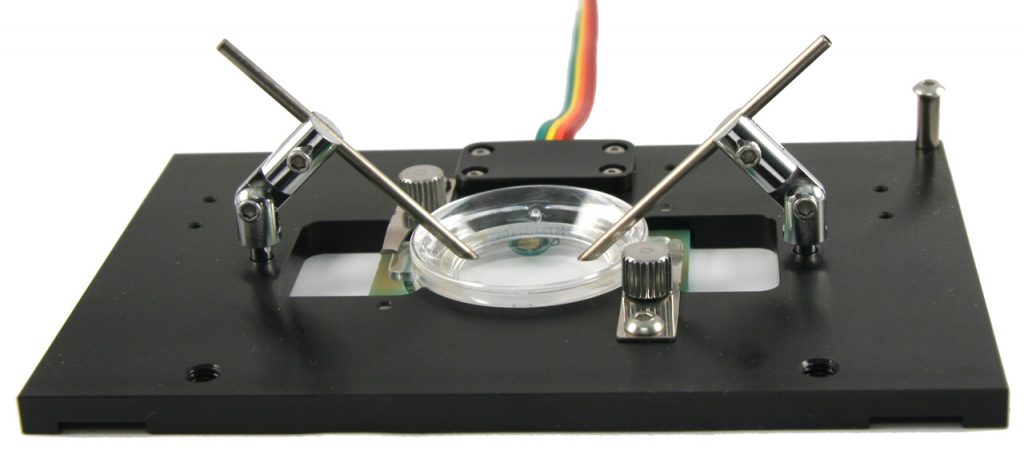
Let’s look at the physical constraints of your experiment. Since you need direct physical access to your specimen you will need an open dish system. You will want the highest resolution images you can get so the specimens should be on a glass #1.5 coverslip. The most efficient means to maintain temperature is direct heating of the coverslip where the cells are adhered. Note: you don’t want to heat the stage and should only provide heat to the specimen. In this case your best choice is the Delta T Culture Dish System. It is a coverslip bottomed, open dish micro-environment that heats only the media and specimens in the dish with a very fast, closed loop feedback system to ensure precision temperature control. This system is designed to be used with numerous accessories that customize it to conform to your experimental constraints. The following configuration would be ideal for your experiment: Cells can be plated in a Delta T Dish and have an electrical field introduced to them by a fixture containing bipolar platinum electrodes that are attached to a flip in / flip out support mechanism mounted to a dish retainer. A supplemental Hinged Perfusion Adapter is used to support perfusion needles for irrigating and aspirating the dish to facilitate long term-experiments. The hinged accessories allow you to easily exchange dishes, maintain registration and re-establish your desired conditions.

If you are imaging with a high numeric aperture objective, which requires an optical coupling medium, you will also need to warm the objective to prevent it from acting as a thermal sink to your specimen. When warming an objective it is critical to apply heat to the most efficient thermally receptive location on the objective and incorporate the objective as part of the control loop. These characteristics are exclusive to the Bioptechs Objective Heater to prevent overshoot and establish accurate control. To reliably and continually perfuse the dish the Delta T micro-perfusion pump is highly recommended. It will deliver media to the dish at a rate slower than it is removed so the dish cannot overflow. You simply adjust the height of the pickup needle to the level you want in the dish. This simple configuration is easy to set up, easy to maintain and will offer years of reliable data collection.